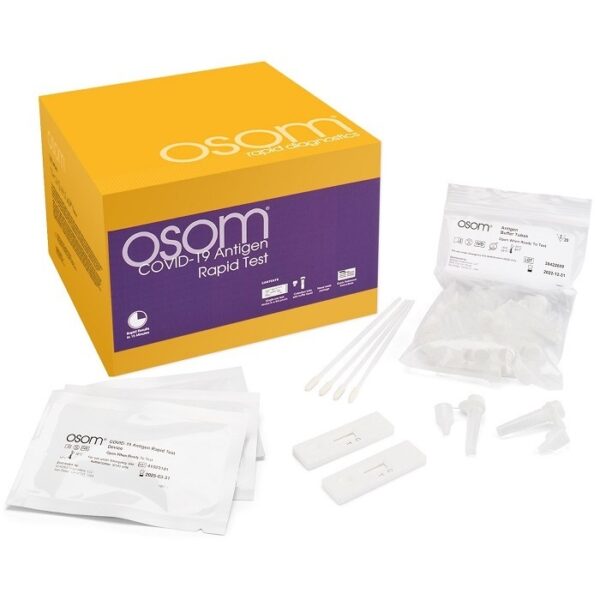
OSOM COVID-19 Antigen Rapid Test Kit EUA 40/Box Respiratory Test
OSOM COVID-19 Antigen Rapid Test Kit EUA 40/Box
Notice – This item is not returnable
Sekisui Respiratory Test Kit OSOM COVID-19 Antigen Rapid Test EUA 40/Box, The OSOM® COVID-19 Antigen Rapid Test is a lateral flow chromatographic immunoassay intended for the qualitative detection of the SARS-CoV-2 nucleocapsid protein antigen in direct mid-turbinate (MT) nasal swab specimens collected by a healthcare provider from individuals suspected of COVID-19 within the first 7 days of symptom onset.
OSOM COVID-19 Antigen Rapid Test Kit EUA 40/Box
Notice – This item is not returnable
Sekisui Respiratory Test Kit OSOM COVID-19 Antigen Rapid Test EUA 40/Box. The OSOM® COVID-19 Antigen Rapid Test is a lateral flow chromatographic immunoassay intended for the qualitative detection of the SARS-CoV-2 nucleocapsid protein antigen in direct mid-turbinate (MT) nasal swab specimens collected by a healthcare provider from individuals suspected of COVID-19 within the first 7 days of symptom onset.
Contents Include:
40 – OSOM COVID-19 Antigen Test Cassette/Devices containing LFI Test Strip in Plastic Housing
2 X (20) OSOM Antigen Buffer Tubes (Nasal swab specimen collection & dispensing tube containing OSOM Antigen Buffer
40 – Sterile, Nasal Swabs
1- Instructions For Use (IFU)
1- Quick Reference Guide For Direct Nasal Swab Samples
Features:
OSOM Covid-19 Antigen Rapid Test is for use under an FDA EUA: https://www.fda.gov/medical-devices/covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2
OSOM® COVID-19 Antigen Rapid Test is a lateral flow chromatographic immunoassay intended for the qualitative detection of the SARS-CoV-2 nucleocapsid protein antigen in direct midturbinate (MT) nasal swab specimens from individuals who are suspected of COVID-19
Qualitative detection of the SARS-CoV-2 nucleocapsid protein antigen in direct mid-turbinate (MT) nasal swab specimens collected by a healthcare provider from individuals suspected of COVID-19 within the first 7 days of symptom onset when tested at least twice over three days with at least 48 hours between tests and from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested at least three times over five days with at least 48 hours between tests
For laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests
This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine patient infection status
Positive results do not rule out bacterial infection or co-infection with other viruses
Laboratories within the United States and its territories are required to report all results to the appropriate public health authorities
All negative results are presumptive and confirmation with a molecular assay, if necessary for patient management, may be performed
Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control measures such as isolating from others and wearing masks
More Information/Documentation Per manufacturer:
1- Sales Sheet
2- Factsheet for Patients
3- Factsheet for Healthcare Providers
4- Quick Reference Instructions
5- OSOM® COVID-19 Antigen Rapid Test Package Insert
6- OSOM COVID-19 Antigen Rapid Test FDA EUA Letter
| Qty: | 40 Tests per Box |
|---|