Clarity Covid-19 Antigen Rapid Test Cassettes, CLIA Waived
Notice – This item is not returnable
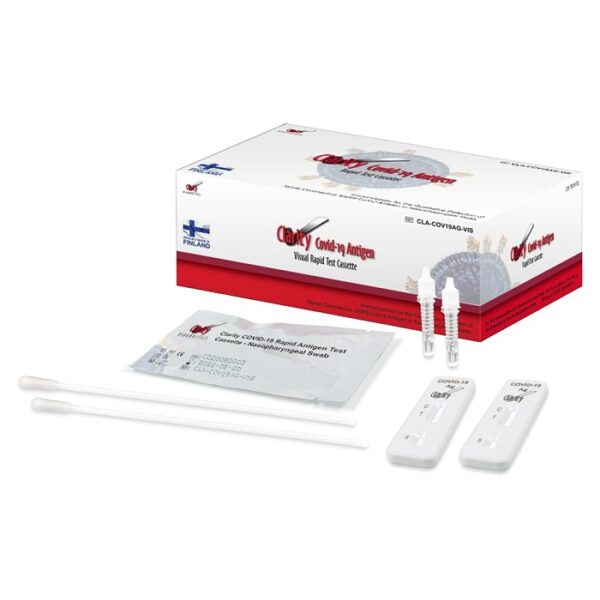
Clarity COVID-19 Antigen Rapid Test Cassettes CLIA Waived
In response to the worldwide pandemic, Clarity is proud to introduce a new line of reliable of COVID-19 products. Our COVID-19 products are Pathway C EUA approved and are proudly manufactured in Finland. With a continuing pandemic, the need for accurate results and reliable tests are of the upmost importance, which is why our COVID products are made to be quick, easy to use, and above all accurate.
Notice – This item is not returnable
Clarity COVID-19 Antigen Rapid Test Cassettes CLIA Waived
In response to the worldwide pandemic, Clarity is proud to introduce a new line of reliable of COVID-19 products. Our COVID-19 products are Pathway C EUA approved and are proudly manufactured in Finland. With a continuing pandemic, the need for accurate results and reliable tests are of the upmost importance, which is why our COVID products are made to be quick, easy to use, and above all accurate.
Product Code: CLA-COV19AG-VIS
CPT Code: 87811QW
Contents:
25 Individually Pouched Test Cassettes
25 Sterile Naso-Pharyngeal Swabs
25 Buffer Tubes
1 Negative Control Swab
1 Positive Control Swab
1 Work Station
1 Package Insert
1 Quick Start Guide
Important Information
This test is not approved for OTC use or At-Home use.
The Clarity COVID-19 Antigen test can be used to test directly collected naso-pharyngeal swab specimens.
The Clarity COVID-19 Antigen test should be ordered for the detection of COVID-19 antigen in individuals who are suspected of COVID-19 by their healthcare provider and who are within six days of symptom onset.
The Clarity COVID-19 Antigen test is authorized for use in laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform high complexity, moderate complexity, or waived tests. This test is authorized for use at the Point of Care (POC), i.e., patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. Our CLIA Waiver can be found on the FDA’s website at the following link: https://www.fda.gov/media/149056/download
More Information/Documentation Per manufacturer:
Clarity CLA-COV19AG-VIS Package Insert
Fact Sheet for Healthcare Providers
Fact Sheet for Recipients
Product SDS
SDS CLA-COV19AG-VIS
Product Brochure
| Qty: | 25/Kit |
|---|