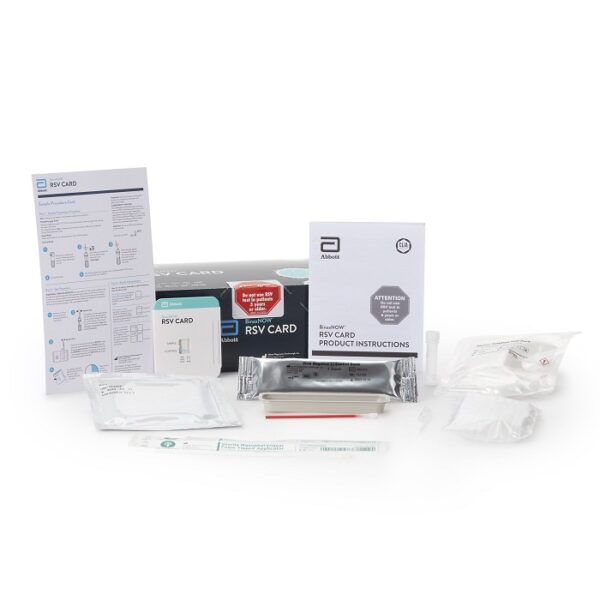
BinaxNOW RSV Rapid Test Respiratory Syncytial Virus Test 22 Tests CLIA Waived
BinaxNOW RSV Rapid Test Respiratory Syncytial Virus Test 22 Tests CLIA Waived
- Alere BinaxNOW® RSV Card is an in vitro rapid immunochromatographic assay used to detect respiratory syncytial virus (RSV) fusion protein antigen in nasal wash and nasopharyngeal (NP) swab specimens from symptomatic patients
- This test is intended for in vitro diagnostic use to aid in the diagnosis of respiratory syncytial virus infections in neonatal and pediatric patients under the age of 5
- Retrospective nasal wash Sensitivity: 89% Specificity: 100%
- Prospective NP swab Sensitivity: 93% Specificity 93%
Brand:Abbott
BinaxNOW RSV Rapid Test Respiratory Syncytial Virus Test 22 Tests CLIA Waived
- Alere BinaxNOW® RSV Card is an in vitro rapid immunochromatographic assay used to detect respiratory syncytial virus (RSV) fusion protein antigen in nasal wash and nasopharyngeal (NP) swab specimens from symptomatic patients
- This test is intended for in vitro diagnostic use to aid in the diagnosis of respiratory syncytial virus infections in neonatal and pediatric patients under the age of 5
- Retrospective nasal wash Sensitivity: 89% Specificity: 100%
- Prospective NP swab Sensitivity: 93% Specificity 93%
More Details
| Brand | BinaxNOW® |
| Manufacturer | Abbott Rapid Dx North America LLC |
| Application | Respiratory Test Kit |
| CLIA Classified | CLIA Waived |
| Contents 1 | (22) Test Cards, Transfer Pipettes, Positive Control Swab, Negative Control Swab, Elution Solution, NP Patient Swabs, Product Insert, Procedure Card |
| Number of Tests | 22 Tests |
| Reading Type | Visual Read |
| Sample Type | Nasopharyngeal Swab / Nasal Wash Sample |
| Specialty | Immunoassay |
| Test Format | Test Card Format |
| Test Kit Type | Rapid |
| Test Method | Lateral Flow Method |
| Test Name | Respiratory Syncytial Virus Test (RSV) |
| Test Type | Infectious Disease Immunoassay |
| Time to Results | 15 Minute Results |
| Item # | 430122 |
| Qty: | 22/Box |
|---|